Can the prosthetic design of an implant prosthesis influence the long-term health of peri-implant tissue? Yes, it can, and this is some fundamental clinical knowledge we have long had from prosthodontics on teeth. Implant Dentistry however has been initially so focused on osseointegration, that it took several decades to awaken to the importance of the prosthesis-tissue interface and how the design of the transmucosal complex can influence both aesthetics and long-term tissue health. This was our main drive to introduce the concept of the Implant Supracrustal Complex some years ago, aiming to study the close interrelation of tissue and components in what we often call as “emergence profile” (1).
The first evidence pointing to this direction came with 3 cross-sectional studies (2-4) which suggested that prosthesis contour of more than 30o as it appears in peri-apical radiographs, is correlated with increased risk for peri-implantitis in bone level implants. Furthermore, convexity of the prosthesis profile has been correlated with increased recession (5), marginal bone loss (6) and peri-implantitis when combined with over contouring of the prosthesis (3). This was the first time that there was an attempt to “quantify” elements of the prosthetic design at implants relate them with pathology. Although these results pointed to a certain direction, it was difficult to extract some concrete suggestions as to what the optimal design should be.
Why was it difficult to come up with specific design guidelines?
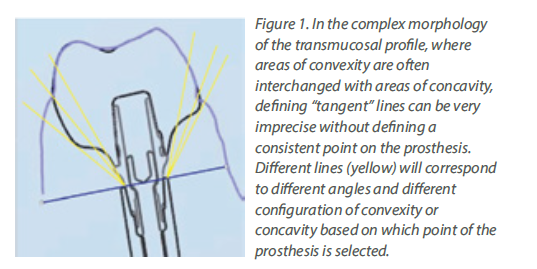
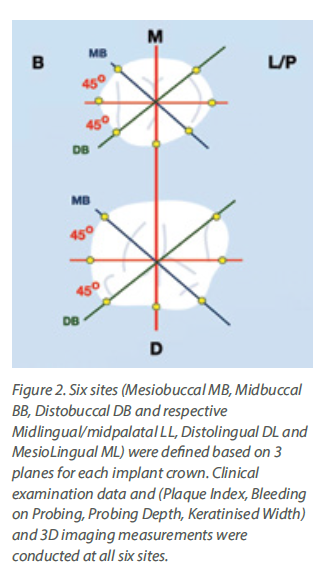
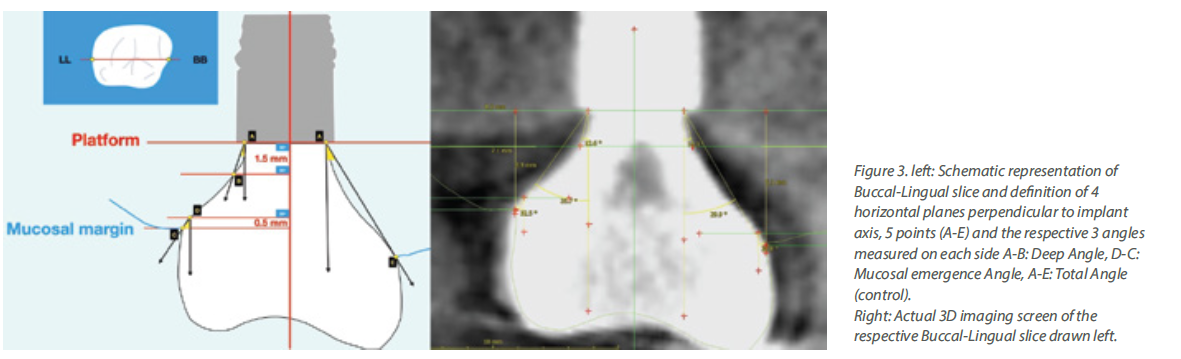
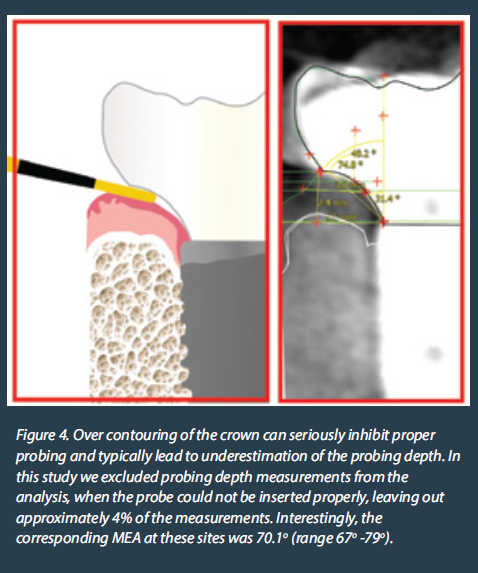
Although a tiny anatomical area, the transmucosal part of the implant-abutment-prosthesis is a highly complex structure. Trying to approach this complex 3-dimensional structure solely by means of the 2-dimensional interproximal projection on periapical radiographs is insufficient. Utilising a periapical radiograph to describe the 360o profile of an implant crown, is like taking a screenshot to describe a movie: it only shows one out of the many very different aspects. Furthermore, a major limitation was that the peri-implant soft tissue is not visible in periapical radiographs, thus there was no possibility to relate the crown contour to the actual position and shape of the healthy or inflamed mucosa. The mesial and distal contour was referred to as “Emergence Angle”, but in reality, there can be no “emergence” without the soft tissue which the crown is emerging from. Emergence Profile (EP) and Angle (EA) based on the Glossary of Prosthodontic Terms 9th Edition, can only be defined in relation to the “circumscribed soft tissues”, which cannot be accounted for in periapical radiographs. Finally, one of the difficulties with previous studies was that the angles were calculated using “tangent” lines. Geometry defines “tangent” as the line which can be drawn perpendicular to the diameter of a perfect circle, but in the complex and irregular curves of the implant crown a precise tangent is next to impossible to define precisely, seriously limiting the reproducibility of measurements (Fig 1). So, although these early studies pointed towards a serious interrelation between prosthetic contour and tissue health, the exact features that affect this relation could not be defined. Also, the threshold of 30o is more of a statistical cut-off point that describes the specific sample, rather than a biological fact. We should note that 2
The first evidence pointing to this direction came with 3 cross-sectional studies (2-4) which suggested that prosthesis contour of more than 30o as it appears in peri-apical radiographs, is correlated with increased risk for peri-implantitis in bone level implants. Furthermore, convexity of the prosthesis profile has been correlated with increased recession (5), marginal bone loss (6) and peri-implantitis when combined with over contouring of the prosthesis (3). This was the first time that there was an attempt to “quantify” elements of the prosthetic design at implants relate them with pathology. Although these results pointed to a certain direction, it was difficult to extract some concrete suggestions as to what the optimal design should be.
Why was it difficult to come up with specific design guidelines?
Although a tiny anatomical area, the transmucosal part of the implant-abutment-prosthesis is a highly complex structure. Trying to approach this complex 3-dimensional structure solely by means of the 2-dimensional interproximal projection on periapical radiographs is insufficient. Utilising a periapical radiograph to describe the 360o profile of an implant crown, is like taking a screenshot to describe a movie: it only shows one out of the many very different aspects. Furthermore, a major limitation was that the peri-implant soft tissue is not visible in periapical radiographs, thus there was no possibility to relate the crown contour to the actual position and shape of the healthy or inflamed mucosa. The mesial and distal contour was referred to as “Emergence Angle”, but in reality, there can be no “emergence” without the soft tissue which the crown is emerging from. Emergence Profile (EP) and Angle (EA) based on the Glossary of Prosthodontic Terms 9th Edition, can only be defined in relation to the “circumscribed soft tissues”, which cannot be accounted for in periapical radiographs. Finally, one of the difficulties with previous studies was that the angles were calculated using “tangent” lines. Geometry defines “tangent” as the line which can be drawn perpendicular to the diameter of a perfect circle, but in the complex and irregular curves of the implant crown a precise tangent is next to impossible to define precisely, seriously limiting the reproducibility of measurements (Fig 1). So, although these early studies pointed towards a serious interrelation between prosthetic contour and tissue health, the exact features that affect this relation could not be defined. Also, the threshold of 30o is more of a statistical cut-off point that describes the specific sample, rather than a biological fact. We should note that 2
lesser-known cross sectional studies which followed similar methodology did not confirm the correlation of >30o contour and peri-implantitis (7,8).